Three-dimensional (3D) imaging plays a vital role in studying how cells are built and how they function. But conventional optical techniques usually require physically holding the cells in place—either by sticking them to a surface or using mechanical tools. This approach doesn’t work well for cells that are freely floating in liquid and can even cause stress that alters their natural behavior. That’s why creating a non-contact, fully optical method to capture 3D images of live suspended cells is still a major challenge for researchers working to better understand biological processes as they happen.
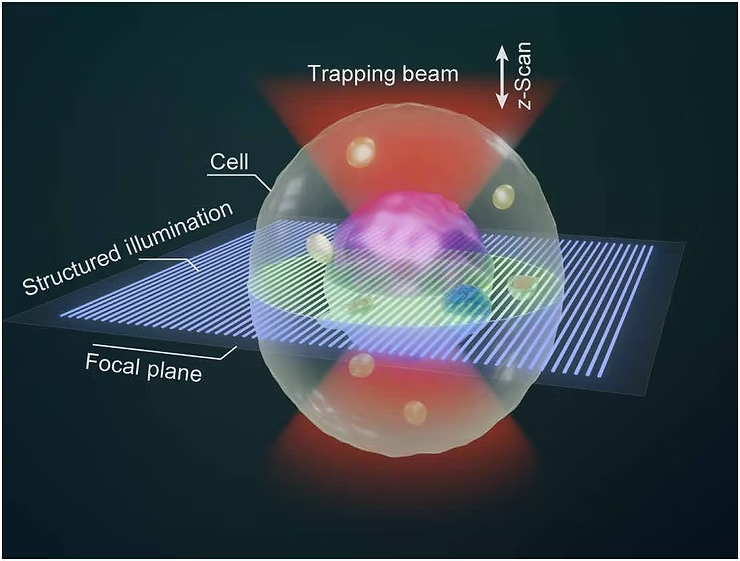
In a recent study published in Science Advances, Prof. YAO Baoli from the Xi’an Institute of Optics and Precision Mechanics (XIOPM), Chinese Academy of Sciences, and Prof. Olivier J. F. Martin from the Swiss Federal Institute of Technology Lausanne introduced a new technique called Optical Tweezer Sectioning Microscopy (OTSM). This innovative method allows researchers to capture 3D images of live cells floating in liquid—without any physical contact. OTSM opens up exciting new possibilities for real-time cell imaging, studying dynamic biological processes, and exploring how cells interact and organize in complex systems.
Researchers created OTSM by combining two advanced techniques: holographic optical tweezers and structured illumination microscopy. Using an array of petal-shaped light traps, they were able to accurately capture and hold multiple live yeast cells floating in liquid. By scanning through different depths and capturing three phase-shifted images at each level, they produced detailed 3D images without needing to physically fix the cells in place.
READ ALSO: Renault tests autonomous bus on Barcelona roads
READ ALSO: Innovative ancient burial site found to be older than Stonehenge
READ ALSO: Using AI, these robots learn complicated skills with startling accuracy
With this method, the team successfully arranged 12 live yeast cells into specific patterns—like hexagons, pentagons, and rings—and captured clear, high-resolution 3D images. The system allows seamless integration between manipulating the cells and imaging them. The HOTs kept the cells stable within the illumination patterns, reducing motion blur and enabling precise vertical scanning for reliable 3D reconstruction.
The reconstructed 3D images clearly showed unique cellular structures, with each cell appearing as a dark outer shell surrounding a bright inner core. On average, the outer shell measured about 4.16 micrometers wide and 6.21 micrometers tall, while the core was roughly 2.72 micrometers wide and 4.34 micrometers tall. The shapes were slightly elongated, with ellipticity values of 0.67 for the shell and 0.63 for the core.
According to Prof. YAO Baoli from XIOPM, the OTSM technology addresses key limitations of traditional bioimaging methods, which depend on fixed samples and mechanical scanning. “This technique brings together structured illumination microscopy and optical manipulation,” he explained, “paving the way for integrating optical tweezers with other imaging technologies to achieve isotropic resolution, wide field imaging, and even super-resolution—essential for advancing modern biological research.